AMOLF researchers unravel how nano-antennas enhance chemical reactions
The chemical industry consumes a lot of energy, not only to initiate reactions but also to separate products from by-products. In a promising emerging field of research, scientists worldwide are trying to use nanoscale antennas to capture and concentrate light into tiny volumes in order to initiate chemical reactions more efficiently and sustainably.
Researchers at AMOLF unraveled how such nanoscale antennas enhance the rate of chemical reactions. They also discovered that using different colors of light can cause completely different chemical reactions to take place.
“This research is still very fundamental, but it shows that it could be possible to design a sunlight powered chemical reactor with these nano-antennas and in which different reactions – and thus different end products – can be chosen. This has potentially huge economic and environmental implications,” says Eitan Oksenberg, a postdoc in the Nanoscale Solar Cells group led by Erik Garnett at AMOLF. They will publish these findings in Nature Nanotechnology on October 4, 2021.
At the interface of chemistry and optics, a new research field has recently emerged that investigates the process of so-called plasmonic photocatalysis. In this process, the exceptional ability of metal nanostructures to concentrate light into sub-nanoscale volumes is used to initiate chemical reactions. “This research is still fundamental, but the concept is very attractive. One reason for that is: many industrial chemical reactions are already catalyzed at the surface of metals”, says Oksenberg. “The idea is that if you concentrate ambient light into very small volumes, you get reaction hot spots in which high temperature or pressure are not needed for an efficient chemical reaction to take place.”
Resolving ambiguities
However exciting it may be, progress in the field is hindered by the ambiguity around the exact mechanism that drives the chemical reaction. Oksenberg: “When nanoscale metal particles are exposed to the right color of light, they act as antennas that capture and concentrate light into a very small volume, which can drive a chemical reaction. Scientists are still debating whether such reactions are driven directly by the concentrated light, by the high energy electrons formed in the metal, or by heat that builds up in the metal when the electrons dissipate their energy.”
Tuning chemical reactions
Oksenberg and his colleagues developed a way to experimentally discriminate between the different possible driving mechanisms. “It is not straightforward to probe what is going on at the surface of metal nanoparticles because the antenna shows a much stronger interaction with light than the molecules that undergo the chemical reaction”, he explains. “However, when the molecules change at the surface of the metal nanoparticle, they cause small changes to the antenna, such as its color and bandwidth. By measuring the reflection of light of more than a thousand individual metal nanoparticles, we can closely monitor these changes over time to get a glimpse into the kinetics of the chemical reaction.”
The researchers expected to be able to discover how exactly chemical reactions are enhanced by metal nano-antennas, but they found that there are several ways. “Even in our very simple chemical system, we saw that different driving mechanisms occur at different colors of light, leading to distinct chemical reactions. This means it is possible to tune the chemical reaction products by choosing the color of the light.”
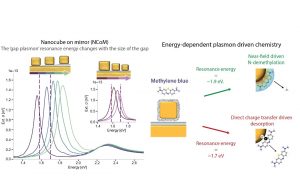
Oksenberg and his colleagues used a configuration of gold nanocubes on a mirror, where a small gap between cube and mirror forms a nano-antenna that concentrates light with a specific color. A molecule called Methylene-blue is bound to the gold particles.
Small variations in the size of both the cubes and the gap result in variations in the antenna color, which has dramatic implications for the chemical reaction that takes place: while bright red light (1.9 eV) cuts off a portion of the molecule, using a slightly darker shade of red (1.7 eV) forces the entire molecule to leave the surface of the metal particle.
Selective chemistry
This discovery is very promising for future applications using metal nanoparticle antennas in chemistry. Oksenberg: “As a scientist, I am excited by the ability to tune a chemical reaction with light and by the richness of the chemistry that we are just beginning to uncover. If we can expand our research to other colors of light outside the visible spectrum, we might even find entirely new chemical pathways that can be triggered with plasmonic resonances. This has the potential to become a disruptive technology. A chemical reactor based on the principles we discovered, is not only very fast and very specific, but also requires very straightforward conditions, like ambient temperature while needing only sunlight as its energy source. The possibility to make the chemical industry more efficient and sustainable with this concept, has huge economic and environmental implications.”
Reference
Eitan Oksenberg, Ilan Shlesinger, Angelos Xomalis, Andrea Baldi, Jeremy J. Baumberg, A. Femius Koenderink and Erik C. Garnett, Energy-resolved plasmonic chemistry in individual nanoreactors, Nature Nanotechnology, DOI will be : 10.1038/s41565-021-00973-6.
Open access link: https://rdcu.be/cyMva
News item obtained from amolf.nl

